Team of international scientists uncover new mechanism in bacterial DNA enzyme opening pathways for antibiotic development
We are thrilled to announce that our latest research on DNA Gyrase has been published in PNAS!
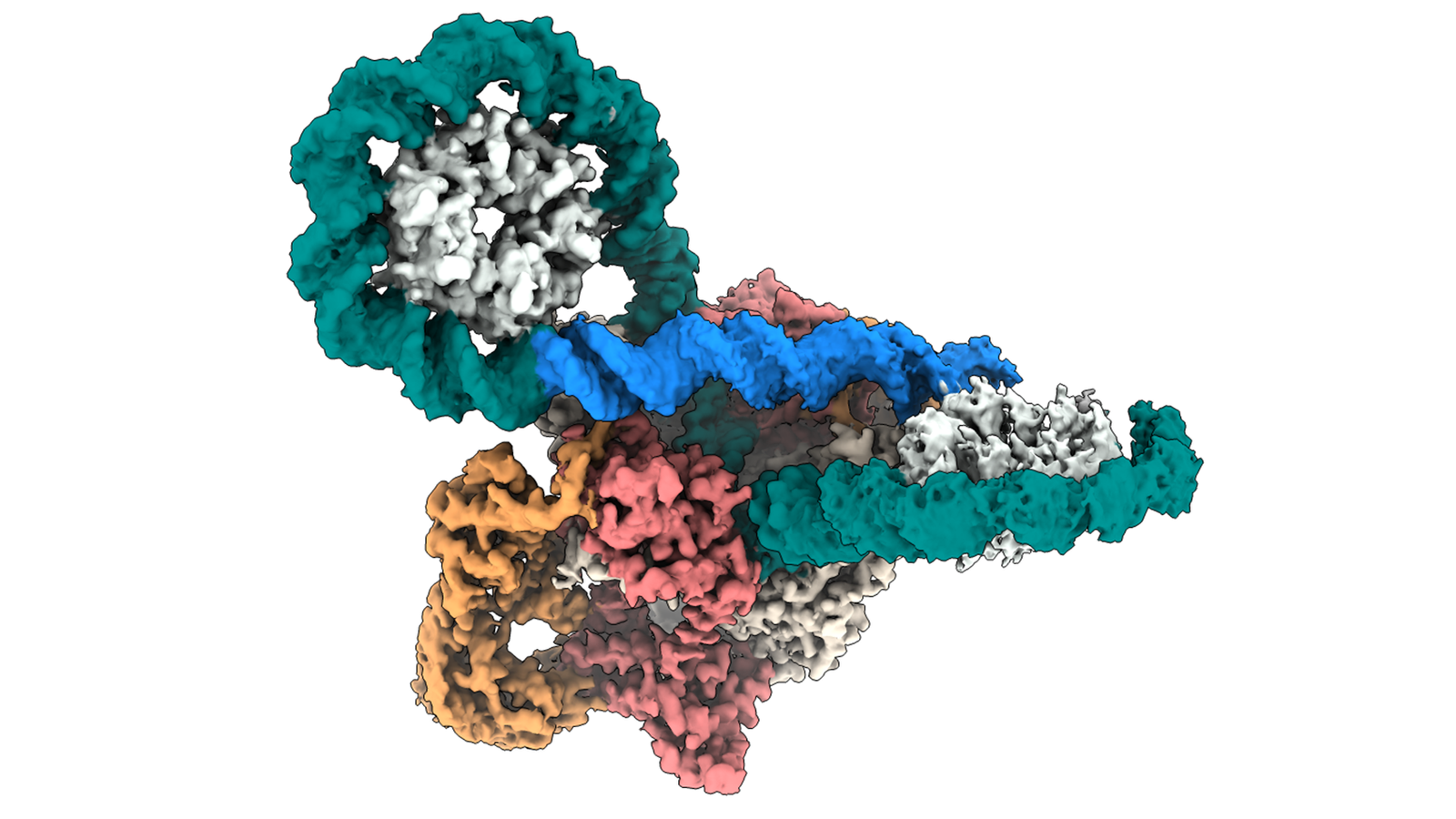
This groundbreaking work explores the mechanism of action of DNA gyrase, a vital enzyme and antibiotic target.
Gyrase functions as a precise nanoscale machine, coordinating an elegant molecular dance between protein and DNA. During a process called supercoiling, gyrase precisely wraps, breaks, threads and re-seals DNA. Exploiting this process, antibiotics such as the fluoroquinolones disrupt the DNA re-sealing, ultimately causing bacterial cell death.
Using high-resolution cryo-electron microscopy, our researchers have uncovered new insights into the “ratchet-and-pawl” mechanism of DNA gyrase. Previously ill-defined, we have presented the first high-resolution structures of a complete E. coli gyrase bound to DNA and in complex with a fluoroquinolone. Our work also describes the protein-DNA interactions of these confirmations, providing new insights into the complex supercoiling process.
These discoveries not only advance our knowledge of bacterial biology but also hold promise for the design of novel antibiotics capable of blocking DNA gyrase in a more targeted way.
Professor Jonathan Heddle commented: “The results suggested the exact position and the order of the complex moving parts of the enzyme during when the supercoiling process occurs were not quite as we previously thought, and this could impact how we design new inhibitors”.

Credit to Sonhita Chakraborty for this lovely image



